January 12, 2026, Guangzhou, China, Creative Biosciences, a leader in fecal DNA–based colorectal cancer screening in China, has released its annual update of real-world application data for Colosafe, the company’s proprietary early colorectal cancer screening test. The annual disclosure reflects Creative Biosciences’ long-standing commitment to transparency, scientific rigor, and the use of real-world evidence to advance public health.
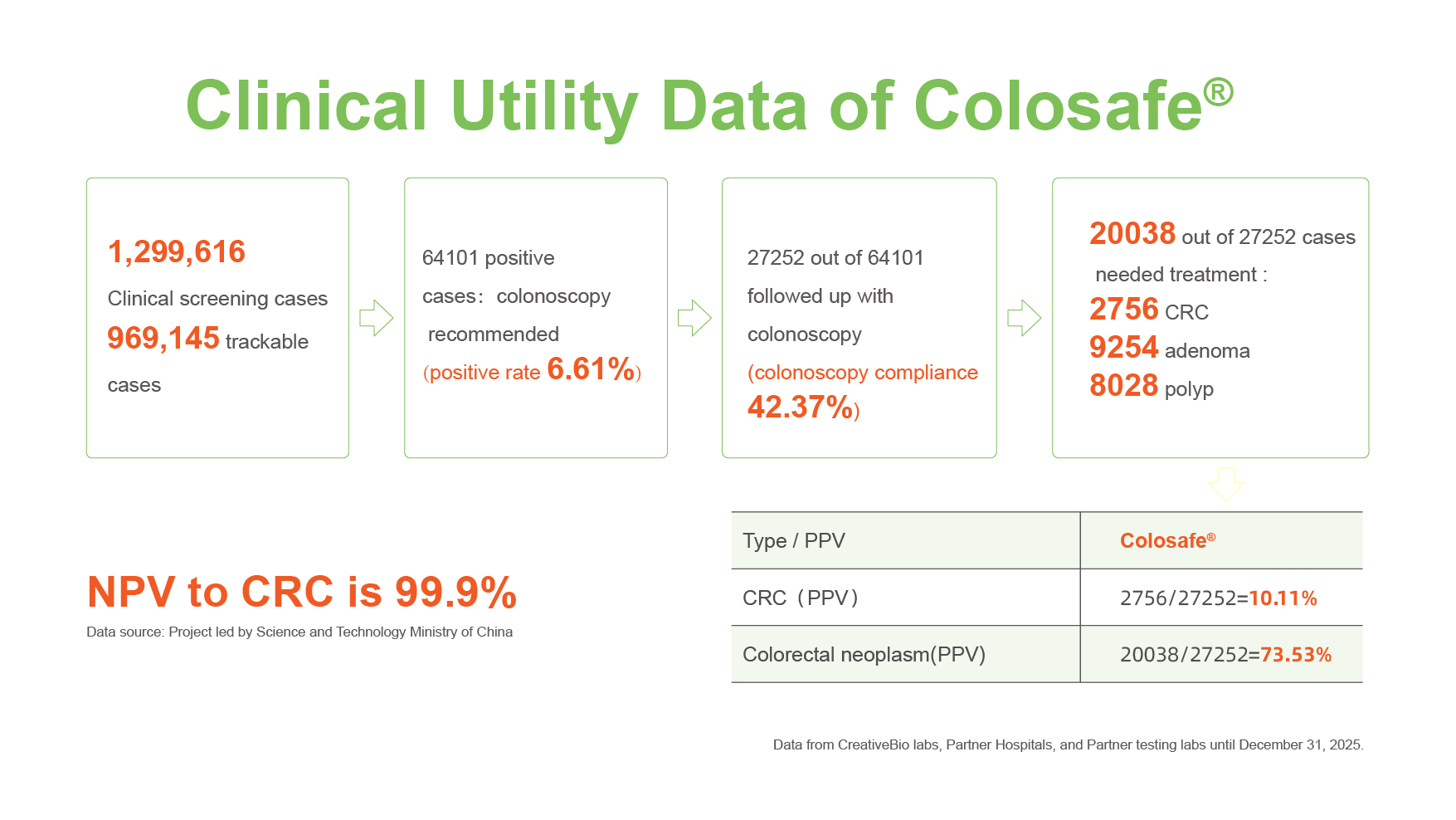
As of December 31, 2025, Colosafe has been utilized in more than 1.29 million screenings. This represents an increase of nearly 300,000 tests compared with the 1 million cases reported in the previous year, highlighting continued growth in clinical adoption and expanding market penetration.
Large-Scale Real-World Evidence Demonstrates Clinical Value
Based on aggregated data from Creative Biosciences’ certified clinical laboratory, participating clinics, and third-party partner institutions, a total of 1,299,616 Colosafe tests have been completed, with 969,145 cases supported by traceable follow-up data. Among these cases:
64,101 tests returned positive results, corresponding to an overall positive rate of 6.61%. All individuals with positive results were advised to undergo follow-up colonoscopy.
27,252 individuals completed colonoscopy, resulting in a follow-up compliance rate of 42.51%.
Colonoscopy and pathological evaluation identified 20,038 individuals requiring clinical intervention, including:
2,756 cases of colorectal cancer
9,254 cases of adenomas
8,028 cases of polyps
Colosafe demonstrated strong diagnostic performance in real-world implementation:
Positive Predictive Value (PPV) for colorectal cancer: 10.11%
PPV for colorectal neoplasm: 73.53%
Negative Predictive Value (NPV) for colorectal cancer: 99.9%
These results confirm Colosafe’s effectiveness as a non-invasive screening tool for large-scale colorectal cancer screening and risk stratification.
CT Value Analysis Enables Risk Stratification
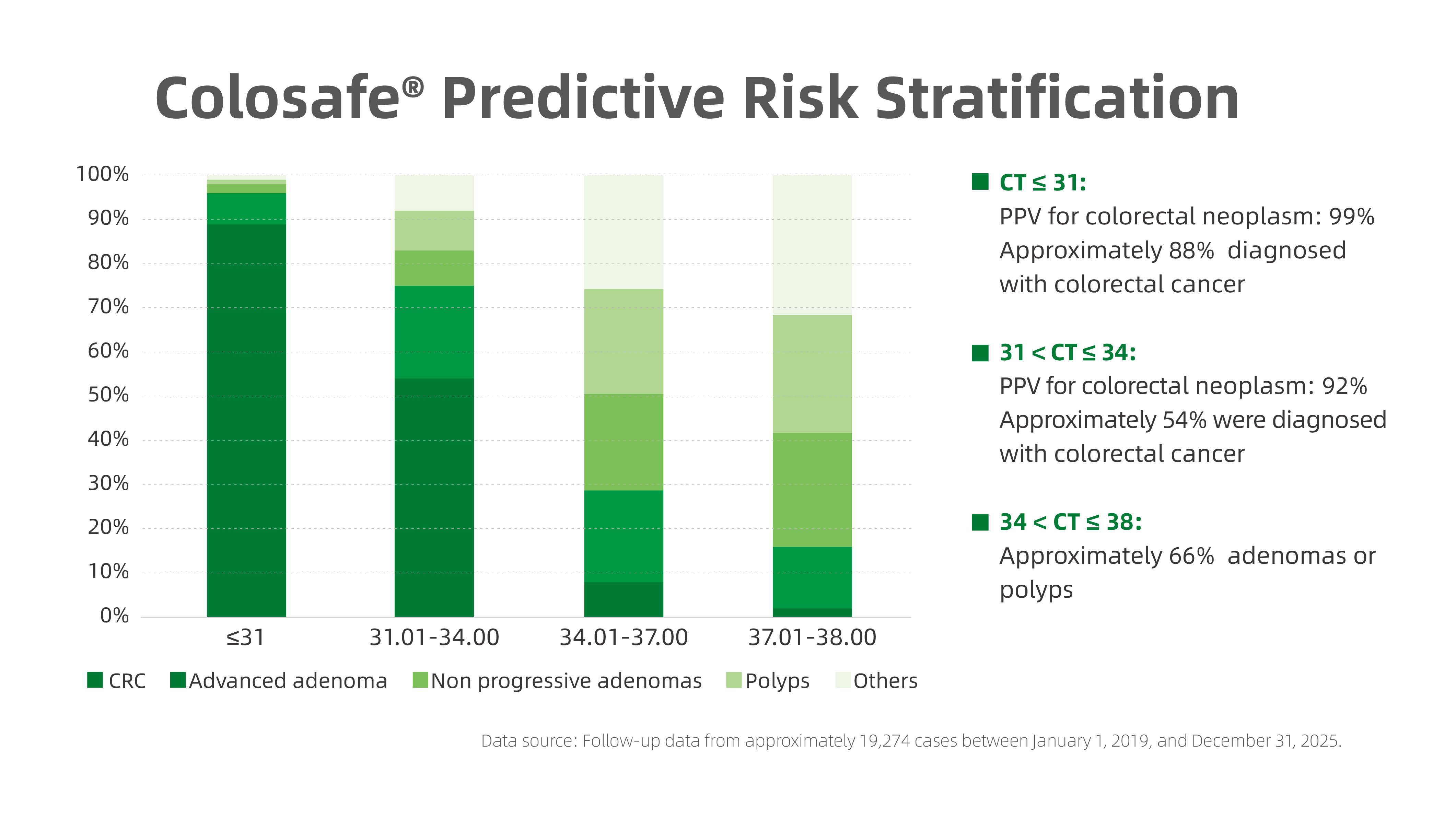
Further analysis of 19,274 Colosafe–positive cases with confirmed colonoscopy and pathology results revealed a clear association between CT values and clinical outcomes:
CT ≤ 31:
PPV for colorectal neoplasm: 99%
Approximately 88% of individuals were diagnosed with colorectal cancer
31 < CT ≤ 34:
PPV for colorectal neoplasm: 92%
Approximately 54% were diagnosed with colorectal cancer
34 < CT ≤ 38:
Approximately 66% were diagnosed with adenomas or polyps
These findings provide strong support for clinical risk stratification, prioritization of follow-up, and tiered patient management, further enhancing the test’s practical value in real-world screening programs.
Founder’s Perspective
Dr. Hongzhi Zou, Founder and Chairman of Creative Biosciences, commented: “1.29 million tests is far more than a statistic. Each result adds to the evidence supporting the stability and reliability of Colosafe in both high-risk populations and routine screening settings. More importantly, every test represents a life at a critical moment. With deep respect for life, we remain committed to rigorous science, continuous innovation, and compassionate care, guided by our mission — Human Health, My Mission — so that more cancers can be detected and treated at a curable stage.”
Broadening the Early Cancer Screening Portfolio
In addition to colorectal cancer screening, Creative Biosciences continues to expand its early detection pipeline. On May 29, 2025, the company received regulatory approval by NMPA for Lunsafe®, its first In-house developed sputum DNA methylation test for early detection of lung cancer. Clinical trial results showed:
Overall sensitivity for Stage I–IV lung cancer: 82.82%
Specificity: 91.79%
Lunsafe provides a non-invasive and convenient option for early lung cancer detection, addressing significant unmet needs in clinical practice.
From Colosafe to Lunsafe, each product reflects a deep understanding of clinical demand and patient needs, while the continuous accumulation of real-world data supports the company’s mission to improve the accessibility and precision of early cancer screening.
Looking Ahead
As real-world evidence continues to grow and the company’s product portfolio expands, Creative Biosciences will remain focused on innovation in early screening technologies for high-incidence cancers. By broadening application scenarios and strengthening localized solutions, the company aims to contribute meaningfully to cancer prevention and control efforts in China and on earth.