Guangzhou, China — October 30, 2025 — Creative Biosciences is accelerating its global growth by expanding across Latin America through new strategic partnerships and the regional introduction of its flagship product, Colosafe®, a noninvasive stool DNA test for the early detection of colorectal cancer.
From October 16–18, Creative Biosciences participated in the 2025 Latin American Congress of Coloproctology in Cartagena, Colombia—the region’s leading professional forum in the field.
Dr. Hongzhi Zou, Founder and Chairman of Creative Biosciences, delivered a keynote presentation titled “Revolutionizing Colorectal Cancer Screening with Stool DNA Testing — Early Detection with Colosafe”. His speech drew strong interest from leading experts and healthcare professionals at the congress.
Dr. Zou delivers keynote at the 2025 Latin American Congress of Coloproctology, Cartagena, Colombia.
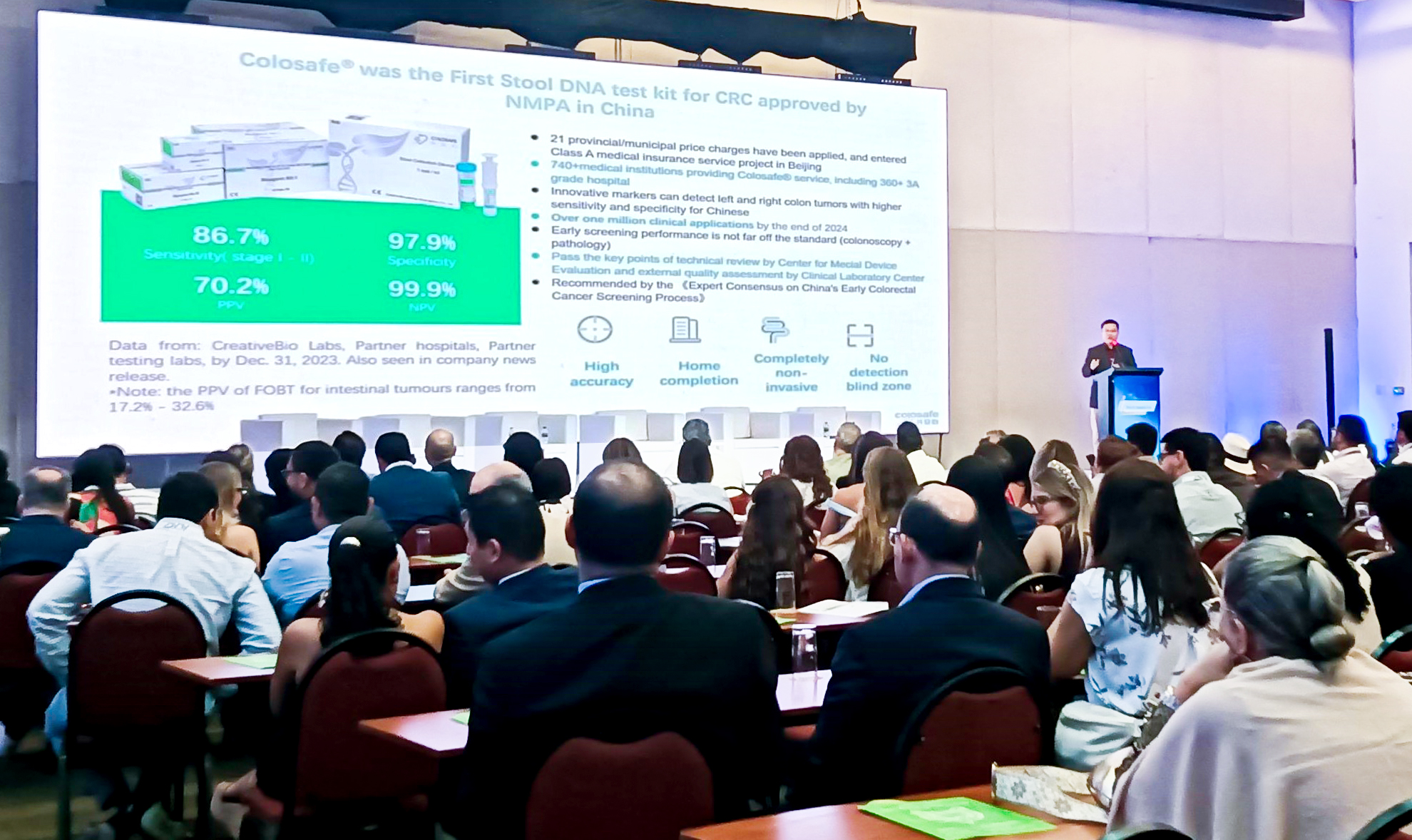
Dr. Zou shared the company’s innovation journey and highlighted the proven performance of Colosafe, the first stool DNA test for colorectal cancer approved by China’s National Medical Products Administration (NMPA) in 2018. Offering 87% sensitivity for early-stage (I–II) colorectal cancer and 98% specificity, Colosafe provides high accuracy, simple at-home sampling, and a fully noninvasive process, outperforming traditional screening methods.
To date, Colosafe has obtained regulatory approval in 46 countries and regions, including the European Union and Brazil, with more than one million tests conducted by the end of 2024. The test has been successfully utilized in large-scale community screening programs across China, helping thousands detect colorectal cancer earlier when treatment is most effective.
At the Cartagena congress, Dr. Zou met with Dr. Carlos Figueroa, President of the Colombian Association of Coloproctology, Dr. David Baquero, Vice President, and other leading specialists. Colosafe earned wide recognition from regional experts for its clinical reliability and potential to improve accessibility to early cancer screening across the region.

Creative Biosciences’ Latin America team introduces Colosafe to physicians at the conference.
Together with its local partner Genproducts (Colombia), Creative Biosciences hosted an exhibition booth where physicians from Venezuela, Panama, Ecuador, Peru, Argentina, Mexico, and Paraguay explored Colosafe’s clinical applications. Following the event, Dr. Zou visited Genproducts’ Laboratorio Genetix in Bogotá to discuss future cooperation and market opportunities in Colombia.
From October 21–24, Dr. Zou continued his Latin American visit to Chile and Brazil, meeting with partners Biosonda and Gentest for research and strategic collaboration.
In Santiago, he visited top medical institutions including Clínica Alemana, Clínica Las Condes, Clínica Universidad de los Andes, Clínica UC-Christus, and Clínica Indisa. Clínica UC-Christus and Clínica Indisa confirmed plans to initiate large-scale clinical studies validating Colosafe and Lunsafe. Lunsafe is a novel noninvasive DNA test for early detection of lung cancer. It is newly innovated by Creative Biosciences and approved by NMPA in May 2025.

Dr. Zou and team visiting leading healthcare institution in Santiago, Chile.
In São Paulo, Dr. Zou joined Mr. Roberto Luiz Guttmann, CEO of Gentest LTDA, and Dr. Marcelo Coutinho, Medical and Scientific Advisor, to host the Colosafe market launch. The event attracted experts from São Camilo Hospital, Hospital das Clínicas of the University of São Paulo, and major laboratories including DNA Club, Centogene, and DB Diagnósticos. Several laboratories signed up for technical training and validation programs, marking a major step forward for Colosafe’s expansion in Brazil.

Dr. Zou presents Colosafe® at the market launch in São Paulo, Brazil.
Latin America and the Caribbean are home to more than 662 million people, yet colorectal cancer screening remains limited to fecal immunochemical tests (FIT) and colonoscopy, leaving a gap between convenience and precision. Colosafe bridges that gap by offering a noninvasive, accurate, and accessible solution for early detection among average-risk populations.
Through continuous collaboration and innovation, Creative Biosciences is deepening its engagement across Latin America. The company remains committed to advancing early cancer detection, empowering local healthcare innovation, and improving outcomes for patients across the region.
About Creative Biosciences
Founded in 2015, Creative Biosciences has envoled a leading biotechnology company dedicated to developing noninvasive molecular tests for early cancer detection. Its flagship products Colosafe and Lunsafe utilizing DNA methylation biomarkers to detect colorectal and lung cancers at an early stage, enabling timely diagnosis and saving lives worldwide.