January 10, 2025, Creative Biosciences (Creativebio) hosted a celebration for Colosafe realizing one million tests at its headquarters in Guangzhou China. Dr. Hongzhi Zou, founder and chairman, presided over the ribbon-cutting ceremony and delivered a speech. Numerous medical experts, partners, and users expressed their encouragement and gratitude, wishing that Colosafe will move forward steadily, make greater contributions to cancer prevention and treatment in China to bring peace of mind to millions of families.

Celebration of one million tests of Colosafe
Professor Wang Jianping, Honorary President of the Sixth Affiliated Hospital of Sun Yat-sen University and President of Qianhai Life Guangzhou General Hospital, sent a message confirming: "Non-invasive stool DNA screening makes it easier for the public to accept, improves user’s compliance, and enhances the quality of colorectal cancer screening." The one-millionth user commented with gratefulness, “I didn’t have any symptoms at all before the precancerous lesion was detected. I couldn’t believe it.” Dr. Zou remarked, “We have persevered to do the right things which are difficult to accomplish. Step by step, we have created the first-class product to benefit the whole society. We are striving to become a world-class molecular diagnostics company."

Dr. Hongzhi Zou, Founder and Chairman of Creative Biosciences
Dr. Zou emphasized in his speech, “Achieving one million tests is a milestone in noninvasive DNA test for colorectal cancer in China, as well as in the whole field of genetic testing for all types of cancer. This marks the most significant moment in the history of CreativeBio. It is also a groundbreaking chapter for the entire industry since Colosafe has been proved in the real world to be accessible and achievable in the mass population screening for colorectal cancer. We will hold firmly to the aspiration of Human Health, My Mission, and endeavor to press forward to achieve ten million tests for Colosafe.
Application data in the real-world practice
According to statistics from CreativeBio’s testing labs, partner hospitals, and third-party institutions, as of December 31, 2024, Colosafe has completed 1,008,753 tests, with 706,522 cases having traceable data. Among these, 46,059 cases were tested positive (a positivity rate of 6.52%) and recommended to follow up with colonoscopy. Out of the positive cases, 18,738 proceeded with colonoscopy (a compliance rate of 40.68%), of which 13,295 cases requiring treatment were identified, including 2,255 cases of colorectal cancer, 5,718 of adenomas, and 5,322 of polyps. The positive predictive value of colorectal cancer was 12.03%, while the positive predictive value for colorectal neoplasm was 70.95%, demonstrating excellent diagnostic performance.
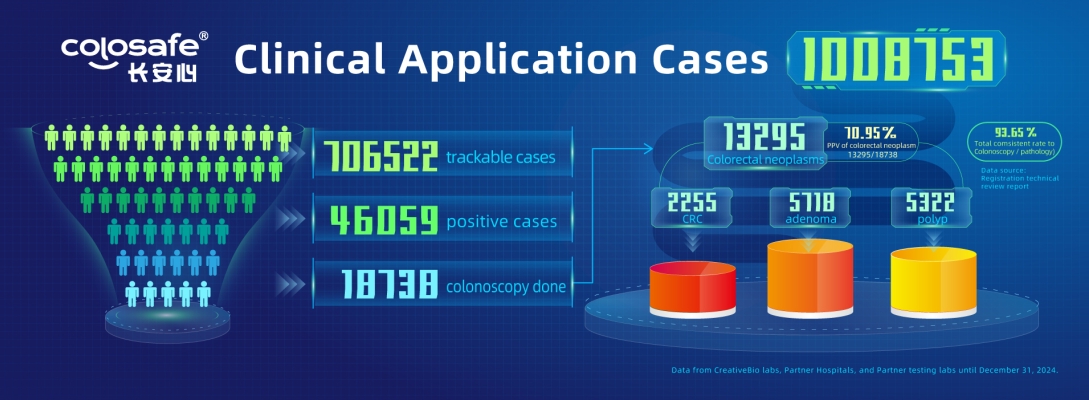
Meanwhile, follow-up data from 13,134 test-positive individuals with traceable colonoscopy and pathology results revealed the following:
- When the Ct value ≤ 31, the positive predictive value for colorectal neoplasm was 99%, with approximately 89% of these individuals diagnosed with colorectal cancer.
- When 31 < Ct value ≤ 34, the positive predictive value for colorectal neoplasm was 92%, with around 57% diagnosed with colorectal cancer.
- When 34 < Ct value ≤ 38, approximately 60% of these individuals were diagnosed with adenomas or polyps.

Colosafe
Colosafe was approved by NMPA in November 2018 with sensitivity of 86.71% and specificity of 97.85%. By far, Colosafe has obtained 24 patents (including 12 invention patents) and national level Scientific and Technological Advancement awards. It has been included in the guidelines because of its high detection performance. The study result from Ten-Thousand Community Population Screening Program for Colorectal Cancer was published as a cover article in the world's top medical journal Gastroenterology in August 2024.
Full Process Quality Control
Colosafe provides outstanding test performance accredited to its superior technology and full process quality control. The automation instruments from sample preprocessing to nucleic acid extraction are all developed in house independently.

Automatic Sample Preprossing and Automatic Nucleid Acid Extraction Instrument
A Multi-Level Prevention and Treatment Model for Colorectal Cancer
Up until now, Colosafe has been utilized in more than 700 top tiered healthcare institutions nationwide in China, establishing decentralized large scale testing network to accomplish early diagnosis and treatment.
Colosafe has not only established an individual screening model in specialty clinics and group screening model in health examination centers but also explored large-scale population screening models at regional and even national levels. Since 2021, large-scale colorectal cancer community screening programs have been implemented in multiple locations, including Huangpu and Zengcheng Districts in Guangzhou, and Shipai Town in Dongguan. Led by the government and supported by collaboration among communities, hospitals, and testing institutions, these initiatives have significantly improved screening participation and colonoscopy compliance. This has led to the establishment of a replicable and scalable new paradigm for colorectal cancer prevention and control.



Sites of colorectal cancer community screening in Huangpu district, Shitan Town in Zengcheng, and Shipai town in Dongguan
These large-scale population screening programs have pioneered a new Colosafe two-step colorectal cancer detection model, combining initial screening + colonoscopy examination. This approach has attained remarkable results, with over 70% colonoscopy compliance for positive cases, colorectal neoplasia detection rate exceeding 80%, and an early diagnosis rate of over 90% for advanced adenomas and early-stage cancers. The screening has demonstrated significant effectiveness and substantial health economic benefits.
In response, Dr. Zou revealed that while a newly developed testing product may show excellent experimental data, its true performance, reliability, and stability must be validated through large-scale population screening. Only with big data can the results be truly convincing. Now, with Colosafe realizing one million tests, its stability and reliability have been proven, making the product a trustworthy solution for early detection of colorectal cancer.
Colosafe gained CE mark in 2020. It obtained registration approval in Brazil in 2024 and Mexico in January 2025. Now, this test has won market entry in forty-two countries and regions covering Asia, Europe, Latin America, and Africa. Starting from Guanzhou, Colosafe has served the whole nation in China and is marching into the global market. It will eventually win the trust from increasing customers all over the world.