January 14, 2025, Colosafe®, a fecal DNA test for early detection of colorectal cancer featured by Creative Biosciences (Guangzhou) CO., Ltd (hereinafter referred to as CreativeBio), gained registration approval by the Federal Commission for the Protection against Sanitary Risks (COFEPRIS) in Mexico. This creates the breakthrough in the globalization for CreativeBio. By far, Colosafe® has obtained market entries in forty-five countries and regions, spanning Asia, Europe, Americas, and Africa where the business partnership has been put in place successfully.

In September of 2024, Colosafe® was proudly exhibited at the 46th Mexican National Clinical Chemists Conference taking place in Mexico City by MCD Servicios Integrales de Diagnósticos, one of CreativeBio’s major partners in LATAM. Experts from clinical laboratories, technical professionals, and distributors from across the country expressed their great interest in Colosafe®. They conveyed full confidence that this novel testing kit would be a game changer in the early detection of colorectal cancer in Mexico.



Colosafe® showcased at the 46th Mexican National Clinical Chemists Conference
Mexico holds strategic position geographically and economically. It has established Free Trade Agreements with many countries including North American Free Trade Agreement (NAFTA) and European Free Trade Association (EFTA), playing an significant role in the international market.
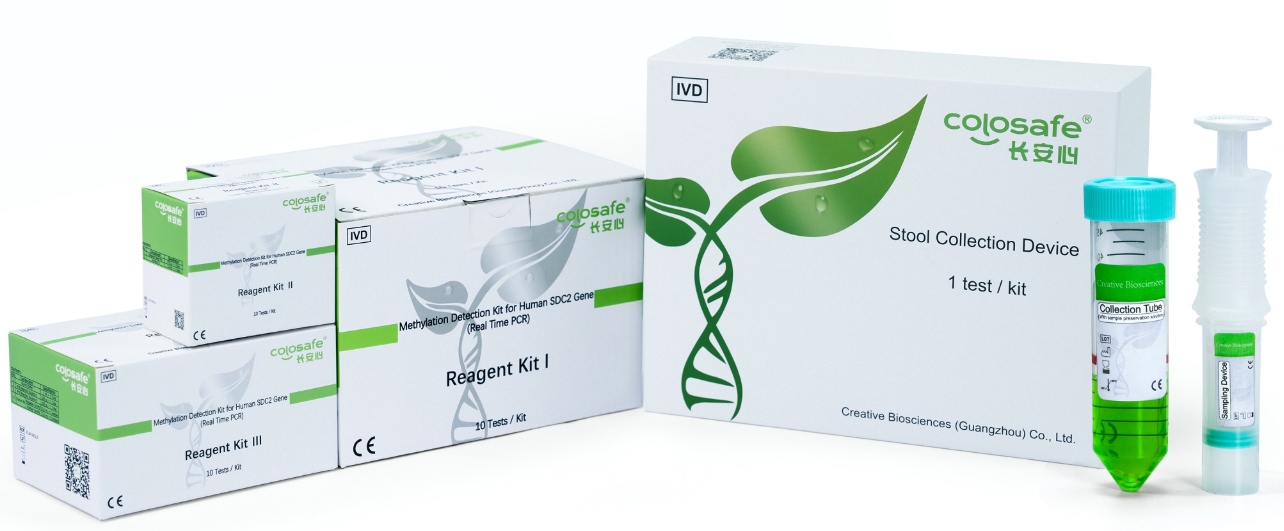
Colosafe® is the first NMPA approved stool DNA test in China. It can precisely recognize the risk of colorectal cancer, providing effective early prevention and treatment with the outstanding performance and convenient sample collection device. Colosafe® has achieved 1,008,753 tests by end of 2024, winning the extensive recognition in the medical practice.
Since 2023, CreativeBio has initiated the strategy of global business development by instituting subsidiaries in Hong Kong China, Seattle USA, and Berlin Germany, covering the key markets in Southeast Asia, the Americas, Europe, and Middle East respectively. These branches have laid solid foundation and support for the global market penetration.
CreativeBio was founded by Dr. Hongzhi Zou who has been dedicated in promotion and application of early cancer detection and diagnosis in China. The company has attracted many outstanding talents from the globe to form a profound research team. On top of Colosafe®, other early detection tests for lung, bladder, liver cancer, and the supporting automated instruments have been developed for more efficient testing.

Automatic Sample Preprocessing and Automatic Nucleic Acid Extraction Instruments
Envisioning the future, all of us at CreativeBio will hold on to our aspiration of “Human Health, My Mission”, and strive to innovate more technology and products in the life science to contribute to cancer prevention for people in the world.