The National "13th Five-Year" Scientific and Technical Innovation Achievement Exhibition took place on Oct 21-27 in Beijing where you could find the most advanced technologies in the nation,like the 600 km/h maglev train, the Sunway Taihulight supercomputer prototype, the Chang 'e-5 lunar probe, etc. With the theme of "Innovation-Driven Development toward a Scientific and Technical Power", the exhibition showcased the major achievements and the latest advancement in scientific and technical development and reform during the 13th Five-Year Plan period.

The exhibition had 12 sectors with total coverage of 21834 sq meters, including review of the past century, basic research, high and new technologies, major projects, agricultural science and technology, social development. Many advanced scientific and technical achievements were exhibited, as well as new achievements in high-quality scientific and technical supporting development.

Colosafe® (Methylation Detection Kit for Human SDC2 Gene and Stool Collection Device), a stool DNA test kit for early detection of colorectal cancer developed by CreativeBio, was the only colorectal cancer screening product at the exhibition. It showed up in “Healthy China” in the sector of “Social Development”, drawing tremendous attention.
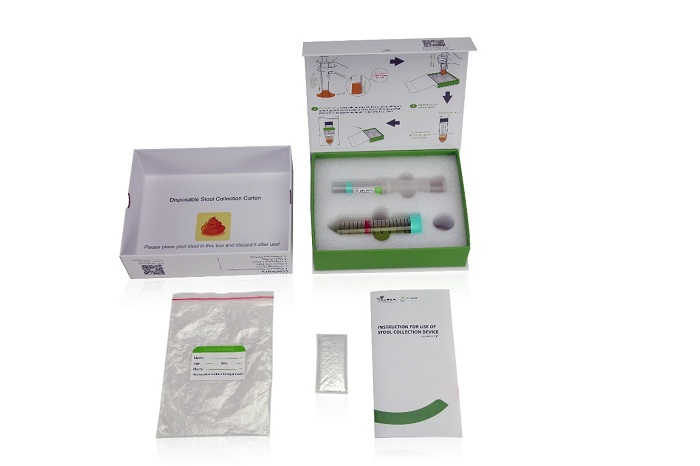
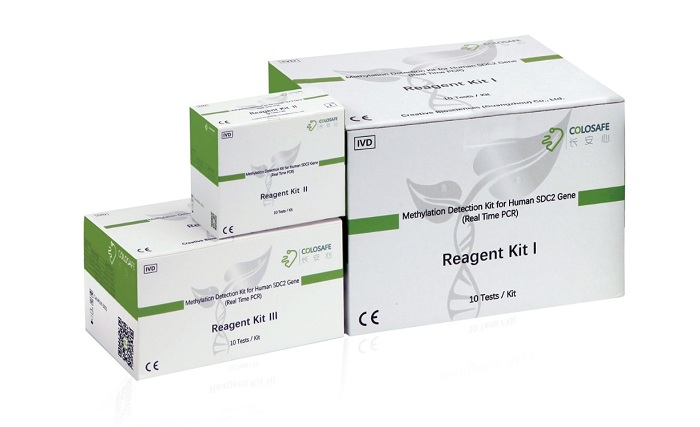

Colosafe® is the first NMPA approved sDNA test kit for CRC in China, which is considered the ice-breaking product in early screening of colorectal cancer.
By far, Colosafe® has been implemented in over 600 healthcare institutions with price codes in 18 provinces and municipal cities. Its performance has been proved superior in detection of precancerous lesion and early colorectal cancer in clinical utility and community screening service, which has won high recognition from the experts, patients, and local governments in terms of major colorectal cancer screening method. Thus, CreativeBio has evidently become the benchmark in the industry.
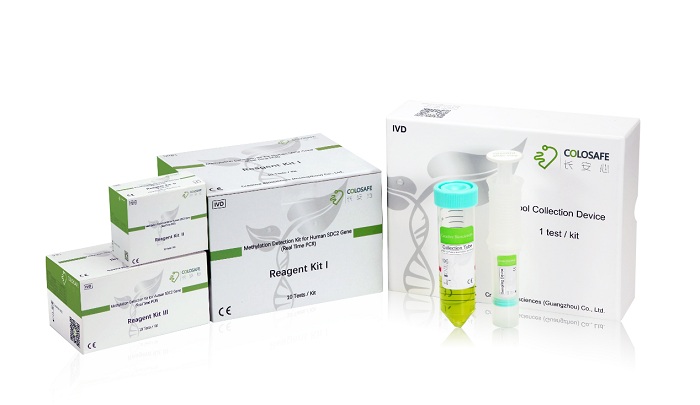
CreativeBio, a leading cancer screening enterprise, participated in the “13th Five Year” Scientific and Technical Achievement Exhibition and demonstrated the accurate sDNA testing for CRC, winning enormous acknowledgement and encouragement. Besides Colosafe®, other early detection test kits for lung, bladder, cervical and liver cancers are in R&D in which surprising advancement has been achieved. The company has also invested enormously in the development of full automation equipment.
CreativeBio has always been adhered to the lofty vision of "Human Health, My Mission" and followed the outline of the “Healthy China 2030” Plan. Under this guidance, CreativeBio is committed to accelerate the speed of research and development, to enhance the level of research and innovation, to help achieve effective cancer prevention and treatment and effective allocation of social medical resources, and to eventually benefit all people.