On May 29, 2022, CreativeBio published its tremendous clinical application data on its annual branding campaign called “Colosafe Day”, carrying on the theme of Love Cannot Wait.

Professor Zhang Beiping, Administrative Director of Department of Spleen and Stomach Diseases and Digestive Endoscopy Center, Guangdong Hospital of Traditional Chinese Medicine; Professor Li Aimin, Deputy Director of Department of Gastroenterology, Nanfang Hospital of Southern Medical University; Professor Kong Xianhe, Deputy Administrative Director of Digestive Endoscopy Department, The Sixth Affiliated Hospital of Sun Yat-sen University; Professor Hongzhi Zou, the founder of CreativeBio; Ms. Deng Xiaoqin, Chief Marketing Officer of CreativeBio; Mr. Leo Liao, Chief Operating Officer of CreativeBio, and other guests were present. Dr. Zhao Jin, Vice President of CreativeBio, was the host of this event.

Dr. Hongzhi Zou making his remark at the event
Blockbuster releasing more than 170,000 prospective clinical utility data and the decentralized business model bringing forth remarkable results.
On the scene, Ms. Deng Xiaoqin, CMO of CreativeBio, officially released the latest prospective clinical utility data of Colosafe® :

Ms. Deng Xiaoqin releasing the latest clinical utility data of Colosafe®
Colosafe®, a stool DNA test kit for early detection of colorectal cancer, has kept revealing authentic clinical application data. By the end of December 2021,there had been over 330,000 Colosafe® tests completed. Among them there were 175,528 clinically traceable cases, of which 12,114 were positive (positive rate of 6.9%), 4970 positive cases followed up with colonoscopy (colonoscopy compliance rate of 41.0%), and 3408 were diagnosed with 755 cases of colorectal cancer, 1444 cases of adenoma, and 1209 cases of polyp. Thus, the conclusion manifested Colosafe® Positive Predictive Value (PPV) to colorectal neoplasia at 68.6% while PPV to colorectal cancer at 15.2%. Another data from screening project sponsored by Ministry of Science and Technology of PRC demonstrated its Negative Predictive Value at 99.9%.
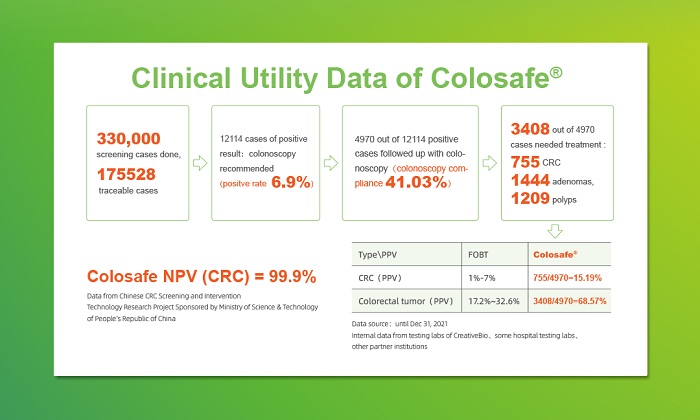
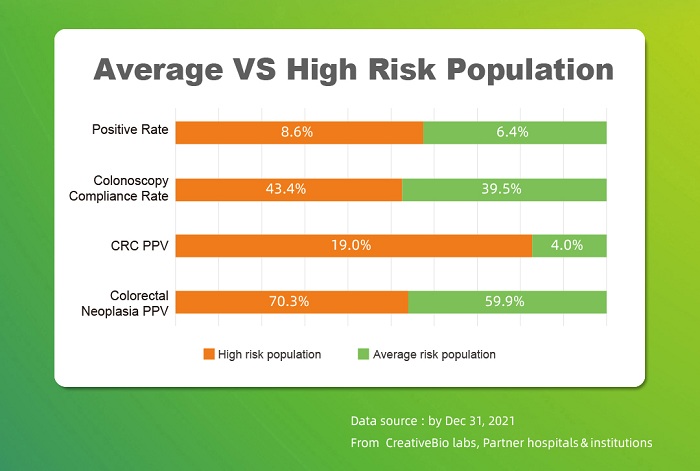
More data of Colosafe® were revealed as well on site:
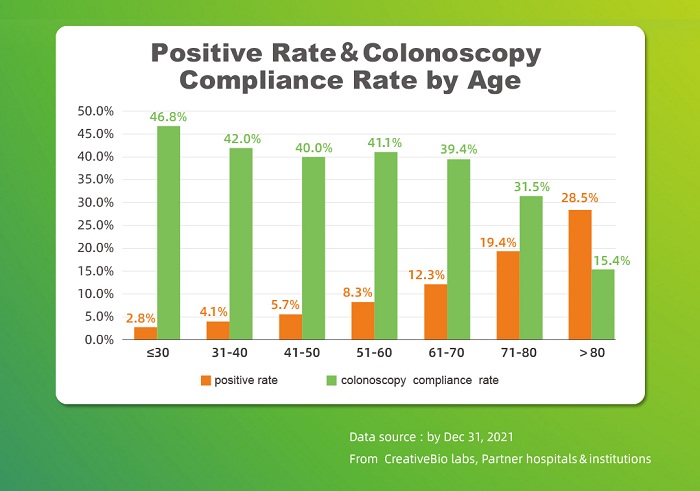
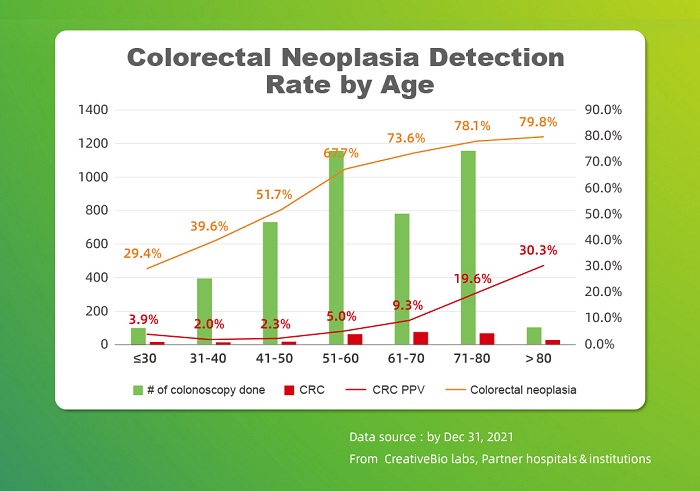
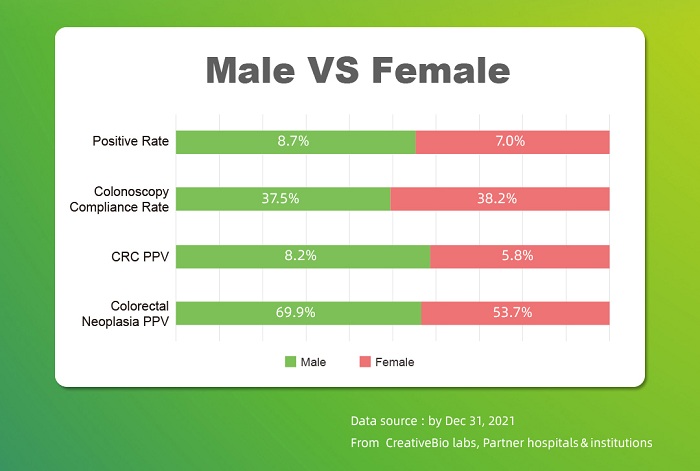
CreativeBio has experienced an unprecedented growth in the past year from 78773 traceable cases in 2021 to 175528 cases in 2022. This data endows benchmark significance for implementing cancer screening products based on serious medical approaches, providing authentic products and data to the industry, and promoting the development of the entire industry market.
Since CreativeBio obtained Colosafe® approval by NMPA, it has launched a comprehensive commercial layout with hospitals as the main channels, and has naturally become the first in the industry to adopt decentralized testing service. Therefore, Colosafe® testig can be performed not only in CreativeBio’s laboratories, but also in partner hospitals and institutions, providing a wider yet feasible landing and coverage.

Professor Li Aimin sharing Colosafe® application data from
Nanfang Hospital of Southern Medical University
At the event, Professor Li Aimin from Nanfang Hospital of Southern Medical University, stressed, “Even the detection rate of early colorectal cancer by colonoscopy has increased year after year, it is only at 15.02% presently. The combination of non-invasive colorectal cancer screening technology represented by Colosafe® and colonoscopy has benefited more and more population.” Prof. Li also shared the prospective application data of Colosafe® from Southern Medical University, demonstrating the effective clinical application progress of stool DNA testing.
Relying on the "decentralized" testing service, colorectal cancer detection products can gain the trust of healthcare institutions and reach more population.
From clinical to community, unlocking new screening scenarios.
Since CreativeBio gained Colosafe® approval by NMPA, it has been actively promoting mass population screening. Colosafe® has been included in the multi-discipline guidelines which endorsed its screening from clinical to community, from hospitals to beyond. Looking back at the year of 2021, CreativeBio has been diligently unlocking new colorectal cancer screening models. It actively participated in the nationwide colorectal cancer screening program for 10,000 people and the community screening in Shipai Town, Dongguan city, making duly contributions to reducing the incidence and mortality of colorectal cancer among community residents and exploring the improvement of colorectal cancer screening.

Prof. Kong Xianhe sharing “Shipai Town Model”
In Dongguan, Colosafe® has created “Shipai Town Model” with its excellent performance in community screening. Prof. Kong Xianhe from the Sixth Affiliated Hospital of Sun Yat-sen University, one of the initiators of community screening in Shipai Town of Dongguan, shared his journey of exploring a new screening model.
“A few years ago we adopted questionnaire + FOBT + plasma tumor markers for screening in Gaobu Town of Dongguan city. In 2021, we combined Colosafe® and colonoscopy for community screening in Shipai Town of Dongguan city. Colosafe® positive results have improved colonoscopy compliance. It has increased the screening accuracy and benefited people.”
Prof. Kong expressed, “The current colorectal cancer screening program in China has some problems in community screening. The application of accurate and convenient stool DNA test is playing an increasingly important role in colorectal cancer screening.”
In 2021, CreativeBio took the initiative to team up with the First Affiliated Hospital of Naval Military Medical University (Shanghai Changhai Hospital) to complete the colorectal cancer screening project for 10,000 people in communities. Thanks to community and the high-quality hospitals with colonoscopy quality control, CreativeBio aims to build a scientific and feasible new model of colorectal cancer screening for community population.

Prof. Zhang Beiping sharing the 10,000 population crc screening project
One participating hospital of this project in Guangzhou is Guangdong Hospital of Traditional Chinese Medicine, which launched online zero contact sample collection device distribution during the pandemic time. Professor Zhang Beiping recalled the situation at that time with deep feelings. From August to December 2021, the recruitment information was delivered through the media, and 641 people signed up online while 630 people returned the samples.The sample recovery rate was 98.3%, making it an unprecedented and successful attempt. The colonoscopy compliance rate of the positive subjects in the 10,000 screening population reached 65%, which was also unprecedented. There were 25 cases of colorectal cancer found from this screening project, reflecting the good screening value.
CreativeBio takes one step at a time and handles every sample seriously with care. They advocate and promote quality control in the industry whether handling the prospective application data of over 170,000 cases or the coverage of 717 healthcare institutions. CreativeBio will hold on to the principle of "Love Cannot Wait" and make commitment to building a firewall of colorectal health for thousands of lives, providing more care and peace of mind to thousands of families.