

Top 100! Creative Biosciences Was Named "The First Guangzhou’s Top 100 Emerging Enterprises"
Early Detection with Colosafe®Creative Biosciences2023.12.27
Early Detection with Colosafe® Creative Biosciences November 21, 2023


Nov 20, 2023, Creative Biosciences (CreativeBio) was invited to join the First Guangzhou’s 100 Emerging Enterprises Integration Innovation Exchange co-sponsored by United Front Work Department of Guangzhou Municipal Party Committee, Municipal Federation of Industry and Commerce, Municipal Bureau of Industry and Information Technology, Municipal SASAC, Municipal Bureau of Science and Technology.
It is reported that since the launch of “Top 100 Training Program” in March 2023, there have been over 700 enterprises competing. The final top 100 list was determined after several rounds of assessment, research and visits conducted, and comprehensive research and judgment decided by the representatives of the special committee. As a benchmark company in cancer screening in China, CreativeBio stands out among the most innovative representative candidates for its pioneering scientific research to be named "The First Guangzhou 100 Emerging Enterprises".
Source:Guangzhou Daily


Left, Mr. Wei Guohua, Deputy Minister of the United Front Work Department of Guangzhou Municipal Party Committee, Party Secretary of the City Federation of Industry and Commerce
Right, Ms. Zhao Jin, Vice President of Creative Biosciences (Guangzhou) CO., Ltd.

The top 100 emerging enterprises are mainly distributed in the fields of new generation information technology (30), biomedicine and health (25), semiconductor and integrated circuit (10), new energy and new materials (17), and intelligent manufacturing (18). In the above five key industries that the government focuses on, all the winning finalists will be trained and empowered in the future. "Among the new top companies in the field of biomedicine and health, CreativeBio is in a leading position in DNA test for colorectal cancer."

The featured product of CreativeBio is Colosafe® which is a cutting-edge stool DNA test for colorectal cancer, with advantages of being noninvasive, accurate and home sample collection. It can precisely detect and interpret the aberrant messages (human SDC2 methylation) from stool for early detection of colorectal cancer to prevent the development of colorectal neoplasia at primary stage. Thus, the prevention and cure of colorectal cancer is achieved. Besides the test kit for colorectal cancer, CreativeBio has accomplished excellent progress in developing other early detection kits for lung, bladder, liver, and cervical cancer. Tremendous investment has been deposited in development of testing related automation equipment simultaneously.
CreativeBio is a leading domestic molecular diagnostic establishment based in Guangzhou High-tech Industrial Development Zone. The company focuses on the development, production, sales, and related automation equipment of Colosafe®, a stool DNA test kit for early detection of colorectal cancer. Adhering to the mantra of Human Health, My Mission, it has been constantly making breakthroughs and developments in the field of early diagnosis and treatment of cancer as well as prognostic monitoring. At present, the company has unfolded a global strategic layout which is covering North Europe, North Africa, Middle East, Southeast Asia, South America. In addition, CreativeBio owns a highly talented team, international cutting-edge R&D potential and many independent intellectual property rights. It is determined to persevere in transforming scientific research results into productivity.
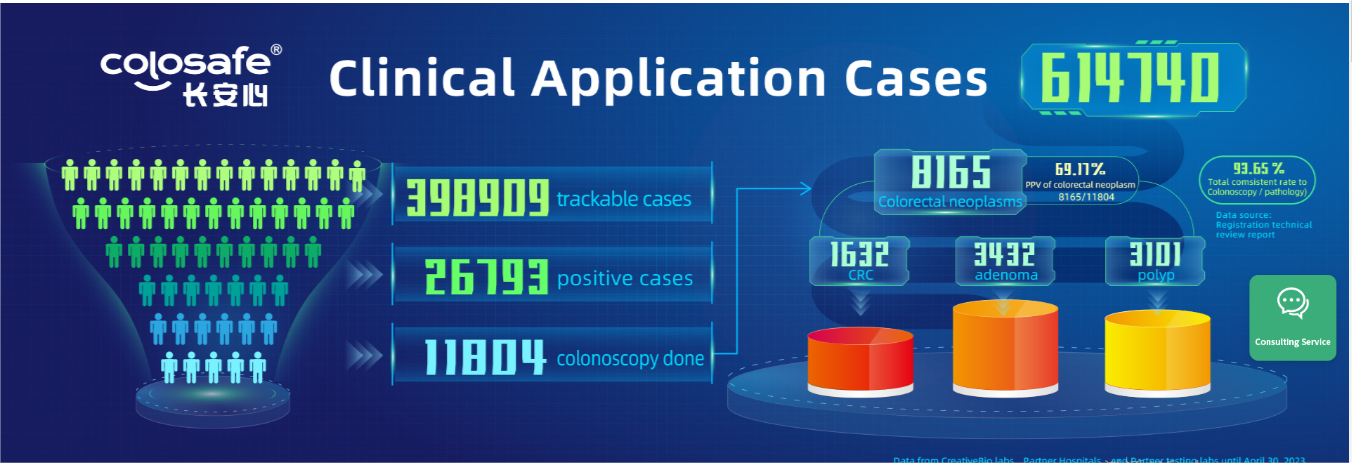
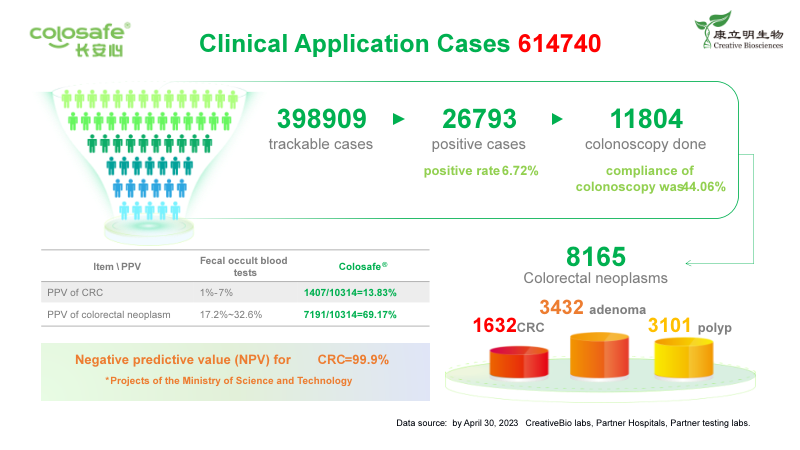
Under the lofty ideal of Human Health, My Mission, CreativeBio has carried forward the social responsibility, elevated the industry standard with the superior performance of Colosafe® for early detection of colorectal cancer, created public health screening model, and increased the population participation to achieve early diagnosis and early treatment of cancer. The data from CreativeBio’s testing labs, some partner and independent labs, has shown that 614,740 Colosafe® tests have been completed with 398,909 trackable cases by April 30, 2023, among which 26,793 positive cases (positive rate of 6.72%) were detected and recommended for colonoscopy follow-up. 11,804 colonoscopy follow-ups were actually performed (colonoscopy compliance rate of 44.06%).
The excellent biopharmaceutical enterprises selected this round not only reflect the vigor and prospect of the industry in Guangzhou, but also inject new impetus into the city’s economic and social development. As a selected enterprise, the reputation and competitiveness of CreativeBio in the industry have also been further demonstrated. The company will take this opportunity to expand investment in research and development, promote technical innovation based on the favorable business environment in Guangzhou, practice benchmark role in the industry, contribute to the driving force of private enterprises, and safeguard the health of the people.
The above English is translated from the Chinese literature published on Yangcheng Evening News and Guangzhou Daily.