Creative Biosciences Successfully Completed the Acquisition of Helixgen
Early Detection with Colosafe®Creative Biosciences2020.03.31

CreativeBio successfully acquired Helixgen in late March, 2020, marking another milestone in its burgeoning history since 2015. This significant merge will enrich the product lines bilaterally by combining CreativeBio’s strong research & development capability in the field of IVD with Helixgen’s engineering development potential. In addition, it allows Helixgen to fully utilize CreativeBio’s sales team and resource channels to promote their products. The powerful alliance of the two companies which complement each other seamlessly will definitely bring the merged enterprise up to a whole new level.
"CreativeBio warmly welcomes Helixgen to join the big family. Helixgen has high quality products in POCT and tremendous expertise in equipment engineering and manufacturing. The merge is like "adding wings to a tiger", making a big leap forward in the development of POCT. CreativeBio is committed to lend full support to ensure Helixgen to grow larger and stronger in every possible aspect." says Dr Hongzhi Zou, founder and CEO of CreativeBio.
The incorporation of Helixgen into CreativeBio will further strengthen its capability of R&D and other areas. Ms. Qing Zeng, CEO of Helixgen and Dr. Tian-Xian Zhao, Vice President of Helixgen, will serve as the Science Adviser and Chief Engineer of CreativeBio respectively. Ms Zeng graduated with a Master of Science degree from the Department of Virology at Sun Yat-sen University in 1990. Since then, she has worked in many institutions including State key laboratory of Viral Genetic Engineering, Institute of Virology, Chinese Academy of Preventive Medicine (Beijing), Cancer Institute of University of Pittsburgh (USA), Johnson & Johnson Pharmaceutical Company (USA), and Schering-Plough (USA). She has gained invaluable experience from her long engagement in the basic research of tumor, viral biomedicine, and development of novel drug and biotechnology. Dr Zhao graduated with a PhD degree in biomedical engineering from Karolinska Institute of Medicine, Sweden. He has worked in the University of Sheffield, Manchester University of Technology, Abbott POCT (Canada), O-Two Medical, Vasogen Inc and other institutions, with more than 20 years of experience in biomedical engineering and medical device industry. They both acknowledge that they are honored to join the big family of CreativeBio and will make full use of their talent to contribute to the long-term development of CreativeBio.

About HELIXGEN (Guangzhou) Co., Ltd.


Immunology Reagent - MHC Tetramer
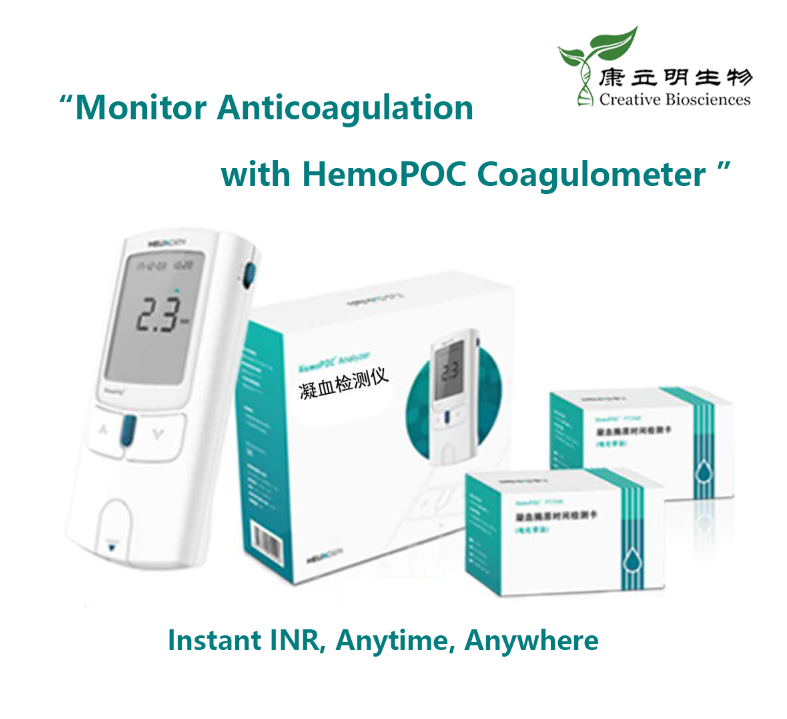
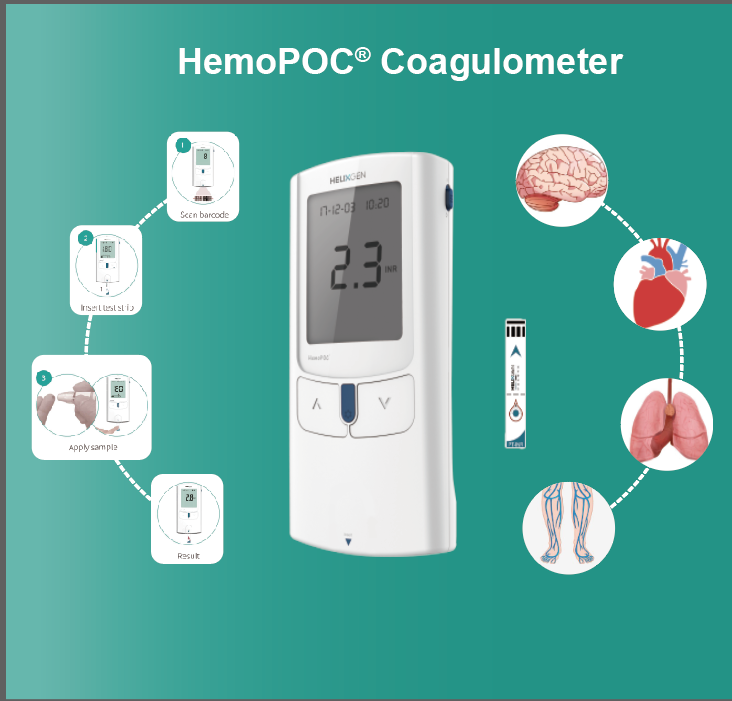
About Helixgen's Molecular Diagnostics POCT Platform


About Creative Biosciences (Guangzhou) Co., Ltd.
CreativeBio is a high-tech bioscience company founded in 2015 by a team of excellent scientists who had returned from abroad. It’s the proud developer, producer and seller of the top brand Colosafe-a stool DNA testing kit for early detection of colorectal cancer. The company is well known for developing detection kits for cancers as well as offering its matching testing services. Headquartered in Guangzhou, CreativeBio has set up offices in Beijing, Shanghai, Xi'an, Hangzhou and established other branches in Hong Kong and Rochester of Minnesota, USA. Its medical testing laboratories scatter nationwide in Guangzhou, Tianjin, Wuhan, Jinan and other places. As of today, the company owns a plethora of independent intellectual property rights and internationally renowned research and development capabilities. Financially, the company has completed several rounds of high-quality venture capital funding and obtained strong support at all levels from the national, provincial, municipal and district governments. It has become a star enterprise in the industry attracting extraordinary attention in China. In line with the lofty ideal of "Human Health, My Mission", we strive to achieve breakthroughs and advancements in early diagnosis, therapeutic treatment, and prognostic monitoring of cancer and other diagnostic fields for the ultimate health benefits of the people.
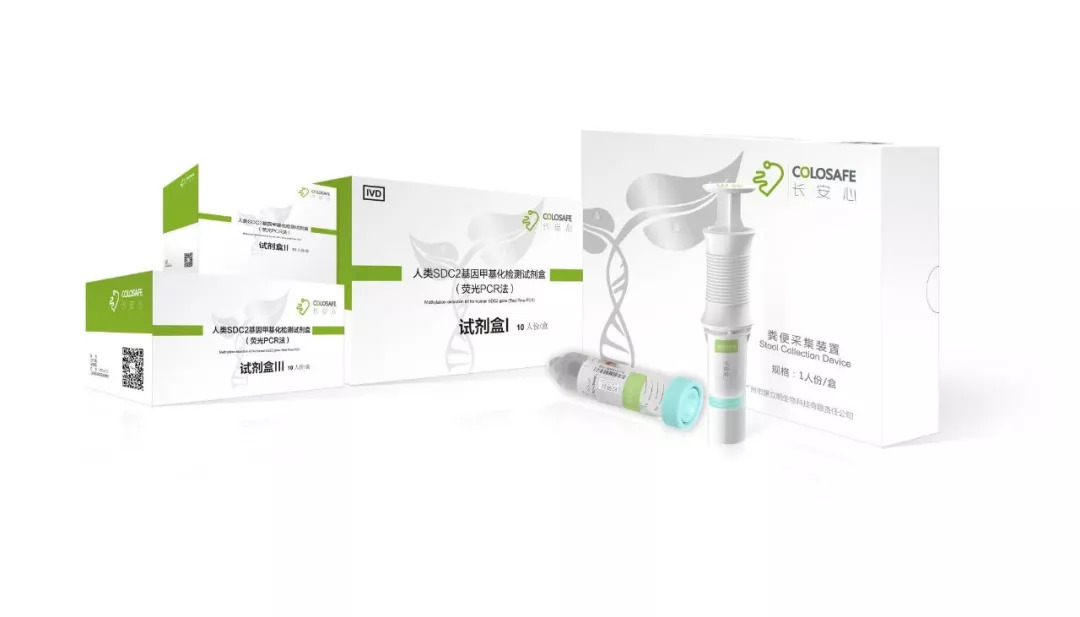
About Colosafe
Colosafe is an early detection tool for colorectal cancer that can accurately interpret abnormal gene changes (human SDC2 gene methylation) in feces. It helps doctors detect patients' colorectal cancer lesions in the early stage, providing effective prevention and treatment of the disease. Colosafe (Detection Kit for Human SDC2 Gene and stool collection device) was approved by National Medical Products Administration (NMPA) on November 20, 2018 and is the only commercially available, NMPA-approved stool DNA test for colorectal cancer in China. In October 2019, Colosafe was recommended in a published expert consensus in Chinese Journal of Medicine as one of the first-line colorectal cancer screening methods. It obtained CE Mark in February 2020, allowing Colosafe to be sold in areas where CE is recognized.